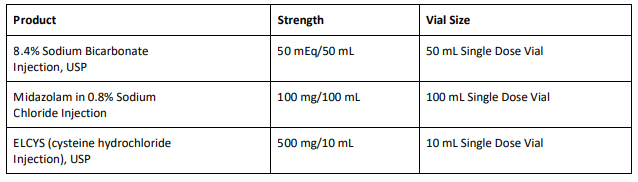
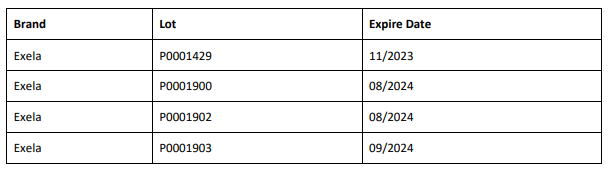
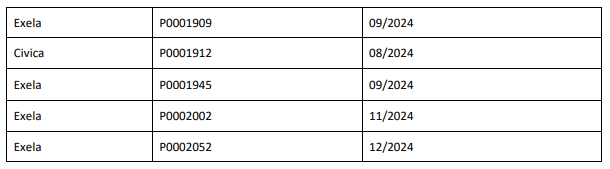
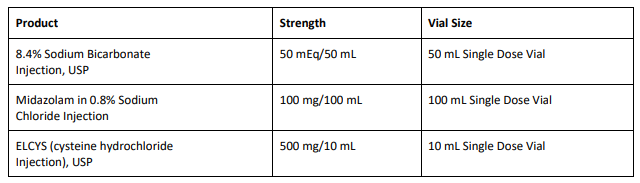
From BeneCard PBF staff
Many clients have seen an increase in utilization of GLP-1 receptor agonists such as Ozempic, Saxenda and Wegovy. As the prescribing trends of these medications continue to grow, we are also seeing increased media coverage warning about the risk of side effects associated with these agents. Benecard is committed to ensuring safe and appropriate utilization of these medications. Here are some important facts that you should know:
What are Glucagon-like peptide 1 (GLP-1) receptor agonists and how do they work?
GLP-1 receptor agonists bind to the GLP-1 receptor and work by mimicking GLP-1, a hormone that’s naturally made by the body. GLP-1 agents stimulate glucose-dependent insulin release which leads to a reduction of blood glucose. GLP-1 agonists also slow the passage of food through the stomach which helps people feel full longer, leading to weight loss.
How are these agents accessible?
GLP-1 receptor agonists have been used to treat type 2 diabetes for more than 15 years and for the
treatment of obesity for eight years.
• Saxenda (liraglutide) and Wegovy (semaglutide) are GLP-1 receptor agonists that are FDA approved for chronic weight management.
• Ozempic (semaglutide, injectable), Rybelsus (semaglutide, oral), Trulicity (dulaglutide), and Victoza (liraglutide) are GLP-1 receptor agonists that are FDA approved for patients with type 2 diabetes.
• Mounjaro (tirzeptatide) is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist (GIP/GLP-1 RA), which is FDA approved for patients with type 2 diabetes.
What are the risks associated with utilization of GLP-1s?
• The most common side effects are gastrointestinal, including nausea, diarrhea, and vomiting. These adverse effects are generally mild to moderate for most patients and improve over time. GLP-1s cause a delay in gastric emptying, but in rare cases, this has resulted in more severe side effects such as gastroparesis (stomach paralysis) and cyclic vomiting. Gastroparesis is a condition that affects the nerves and muscles of the stomach and leads to food sitting in the stomach for too long.
Gastroparesis can have many causes, including diabetes, which makes it difficult to determine how much of a role these drugs play in affecting patients who have reported these side effects. It is believed that reports of extreme side effects such as gastroparesis could be a result of the drug worsening existing “slow stomach” that patients may have but have been unaware of. The FDA says that the benefits of the medication may still outweigh the risks even for this population. Due to the known delay in gastric emptying that GLP-1s cause, the American Society of Anesthesiologists has recently released interim guidance that suggests that patients who are undergoing elective surgery should stop taking these medications at least 1 week prior to surgery, to reduce the risk of nausea, vomiting, and aspiration while under anesthesia.
• GLP-1s also include a warning about suicidal behavior and ideation. The prescribing information for these agents includes a recommendation that patients should be monitored for depression or suicidal thoughts and that therapy should be discontinued if symptoms develop. It is important that patients and prescribers are aware of this recommendation to ensure that they are aware of signs and symptoms of depression, if they should occur.
• Social media influence and celebrities who are promoting the use of GLP-1 agents in addition to drug shortages have contributed to an increase in off-label use and unauthorized versions of these drugs. The FDA has warned that there are potential safety risks associated with using “compounded” GLP-1s, which are now gaining popularity.
• GLP-1s are contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Patients taking GLP-1s should be monitored for symptoms of hypoglycemia, acute pancreatitis, gallbladder disease, and kidney injury.
Benecard’s Continued Commitment to Patient Safety
BeneCard PBF now conducts clinical reviews on all GLP-1 medications to ensure that the appropriate medication is being prescribed for its intended FDA approved use. GLP-1 agents for the treatment of obesity require initial clinical review and continued reauthorizations to ensure that the appropriate dose titration schedule is being followed and that patients are being monitored for unwanted side effects.
Reviews of updated safety data and recommendations are conducted so that, as new safety concerns for these agents arise, we can add these new monitoring parameters or warnings to our clinical review policies. Patient safety and quality healthcare for the patient are always our top priorities.
References
Goodman, B 2023, ‘They took blockbuster drugs for weight loss and diabetes. Now their stomachs are paralyzed’, CNN Health, 25 July, accessed 15 August 2023, They took blockbuster drugs for weight loss and diabetes. Now their stomachs are paralyzed | CNN
Redmond, K 2023, ‘Potential Wegovy, Ozempic link to suicidal thoughts prompts EU investigation’, NJBIZ, 14 July, accessed 15 August 2023, Potential Wegovy, Ozempic link to suicidal thoughts prompts EU investigation – NJBIZ
Constantino, A 2023, ‘People should stop taking Ozempic, Wegovy obesity drugs before surgery, doctors group says’, CNBC, 30 June, accessed 15 August 2023, Stop taking Ozempic, Wegovy before surgery: Doctors (cnbc.com)