Exela Pharma Sciences, LLC, (Exela) is voluntarily recalling the products listed in the table below to the consumer level. Particulate matter identified as silicone was observed during routine inspection of retain samples.
Risk Statement: Administration of an injectable product that contains particulate matter may result in local irritation or swelling in response to the foreign material. If the particulate matter reaches the blood vessels it can travel to various organs and block blood vessels in the heart, lungs or brain which can cause stroke and even lead to death. Exela has not received any reports of adverse events related to this recall.
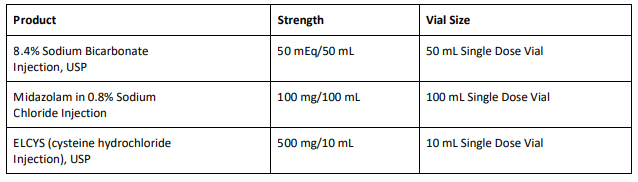
8.4% Sodium Bicarbonate Injection USP is used for treatment of metabolic acidosis and is packaged in a 50 mL glass single dose vials, 20 vials per carton Exela brand (Carton NDC: 51754-5001-5; Vial NDC: 51754- 5001-1, Figure 1) and 25 vials per carton Exela brand (Carton NDC: 51754-5001-4; Vial NDC: 51754-5001-1) and Civica brand (Carton NDC: 72572-740-20; Vial NDC: 72572-740-01, Figure 2).
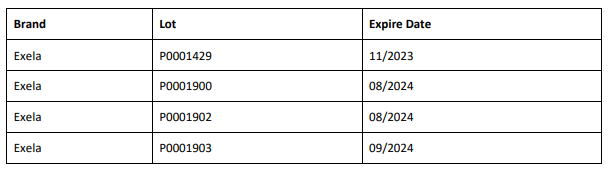
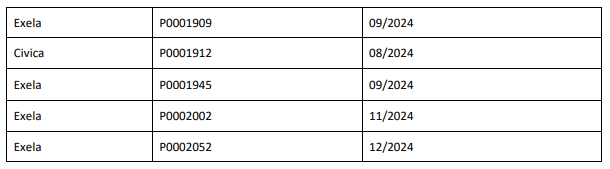
The affected 8.4% Sodium Bicarbonate Injection, USP, 50 mEq/50 mL lots (covering both Exela and Civica brands) include the following lot numbers and expiration dates:
Product was distributed nationwide to wholesalers, distributors, and health systems between January 18,
2022, and February 15, 2023.
Midazolam in 0.8% Sodium Chloride Injection is used for sedation and is packaged in a 100 mL glass vial, 25 vials per corrugated shipper. The vials are labeled with Exela brand (Carton NDC: 51754-2131- 4; Vial NDC: 51754-2131-1, Figure 3).
The affected Midazolam in 0.8% Sodium Chloride Injection 100 mg/ 100 mL include the following lot number and expiration date:

Product was distributed nationwide to wholesalers, distributors, and health systems between July 14, 2023, and September 26, 2023.
ELCYS (cysteine hydrochloride Injection) is used for nutritional requirements per total parenteral nutrition (TPN) and is packaged in a 10 mL glass vial, 10 vials per carton. The vials are labeled with Exela brand (Carton NDC: 51754-1007-3; Vial NDC: 51754-1007-1, Figure 4).
The affected ELCYS (cysteine hydrochloride Injection), USP 50 mg/mL includes the following lot number and expiration date:

The product was distributed nationwide to wholesalers, distributors, health systems, and compounders between July 20, 2023, and August 1, 2023.
Exela is notifying its customers by e-mail and certified mail and is arranging for return and replacement of all recalled product directly to Exela. Customers that have product, which is being recalled should discontinue use, segregate the recalled product, submit a recall stock response form to Exela (even if there is no product to return), and hold the product until shipment instructions are provided by Exela. Customers with questions regarding this recall can contact Exela by phone (828-341-6118) or email at recall@exela.us Monday through Friday, 9:00am – 5:00pm ET. Consumers should contact their physician or healthcare provider if they have experienced any problems related to the usage of this drug product.
(No BeneCard members were affected by this drug recall.)
FDA Reporting
Adverse reactions or quality problems experienced with the use of this product may be reported to the
FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
• Complete and submit the report Online: www.fda.gov/medwatch/report.htm
• Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-
1088 to request a reporting form, then complete and return to the address on the pre-addressed
form, or submit by fax to 1-800-FDA-0178


