Teva Pharmaceuticals USA, has initiated a voluntary nationwide recall of specific lots of various strengths of Fentanyl Buccal Tablets CII to the Consumer Level. Teva USA manufactured and labeled these product lots exclusively for Mayne Pharma Inc. under Mayne’s label. This recall has been initiated because safety updates were omitted in the Product Insert/Medication Guide (MG) that are provided with these recalled lots.
Fentanyl Buccal Tablet is an opioid agonist indicated for the management of breakthrough pain in cancer patients 18 years of age and older who are already receiving and who are tolerant to around-the-clock opioid therapy for their underlying persistent cancer pain.
The main safety concern is a potential for incomplete information needed by health care providers and patients regarding safe use of the product. Not following, or not being aware of, the omitted safety updates in the Product Insert/Medication Guide (MG) could lead to life-threatening adverse events; although, based on a Health Hazard Assessment conducted by Teva, the likelihood of the harm occurrence is considered remote. To date, Teva has not received any complaints related to the product labeling.
Teva notified its customer, Mayne Pharma Inc. on April 27, 2023, alerting them that the lots were recalled and requesting that they return impacted product. Instructions for returning recalled product and receiving a credit are given in the Recall letter and Consumer Recall letter released by Teva.
No BeneCard PBF were effected by this recall.
Consumers with questions or concerns should first consult with their health care provider(s). To report an Adverse Event or Quality Complaint, or if you have medical related questions, please use the following contact information:
Medical-related Questions or to report an Adverse Event:
Contact Medical Information at: 888-483-8279 or USMedInfo@tevapharm.com
Live calls received: M – F, 9:00 AM – 5:00 PM Eastern Time; Voicemail: 24 hrs./day, 7 days/week
Product Quality Complaint-related Questions:
Contact Quality Assurance Services: 888-838-2872, option 4
Live calls received: M – F, 9:00 AM – 5:00 PM Eastern Time; Voicemail: 24 hrs./day, 7 days/week
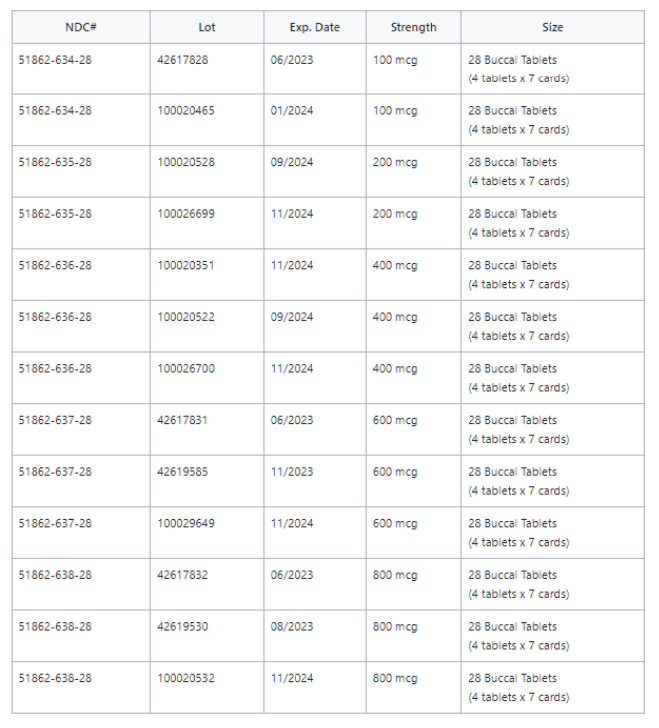
See chart below: